Abstract
Introduction:
The combination treatment of rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone (R-CHOP) became the standard of care first-line treatment for diffuse large B-cell lymphoma (DLBCL) almost 20 years ago. R-CHOP can have both short-term and long-term effects that negatively impact quality of life (QoL), though there is a paucity of research in this area. The phase III REMoDL-B study (ISRCTN 51837425) evaluated the addition of bortezomib to standard R-CHOP therapy in patients with newly diagnosed DLBCL. While the primary endpoint was not met for the study as a whole, follow-up data suggests that bortezomib may benefit some molecular sub-groups. In this secondary analysis of the REMoDL-B study we assessed the impact of R-CHOP on QoL in patients enrolled in the control arm of the study, with 5 years of follow-up.
Methods:
QoL was assessed using EORTC QLQ-C30 version 3.0 and the EORTC chemotherapy-induced peripheral neuropathy module (QLQ-CIPN20). Assessments were performed for patients alive and without progressive disease at the beginning of each treatment cycle, at the end of treatment, and thereafter at months 3, 6, 9, 12, 18, 24, 36, 48 and 60. Two different outcomes from QLQ-C30 were investigated, QL1 and QL2 summary scores, with higher scores reflecting better health-related QoL. Linear mixed models were constructed with responses at each time point nested within participants and a random slope fitted for the effect of time. These models estimated change from baseline for both QL1 and QL2.
Results:
617 patients were enrolled into the R-CHOP control arm of the study. Patients whose gene expression profiling failed (n=158) were not asked to complete QoL questionnaires and were excluded from the QoL analysis. 449/459 patients completed at least one QoL questionnaire to the extent that a QL1/QL2 score could be calculated.
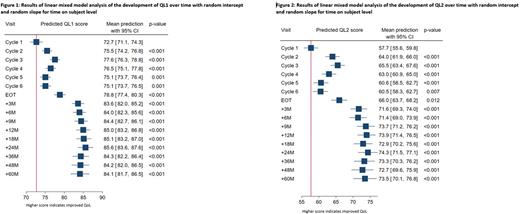
The mean QL1 score was 72.8 at baseline and 86.3 at month 60, whilst the mean QL2 scores were 57.9 and 77.1 respectively, indicating that longer term QoL is not negatively impacted by R-CHOP treatment in those who remain alive and disease-free after 5 years of follow-up. It is notable that QL1/QL2 scores increase as DLBCL regresses during the early cycles of R-CHOP treatment and then drop again in the latter cycles of treatment due to accumulation of chemotherapy toxicity. However, QL1/2 scores remain significantly better than baseline levels (Figures 1 and 2), suggesting symptoms from the disease itself have a greater negative impact on QoL than R-CHOP treatment.
Factors significantly associated with higher QoL across all timepoints of the study included older age, male gender, completion of R-CHOP treatment, and stage II-IV disease. Lower QoL amongst those with stage I disease is not entirely clear, though may be partially explained by the small stage I patient numbers in this group (n=12). Factors significantly associated with lower QoL across all timepoints of the study were the presence of B-symptoms and an IPI score of ≥2 at diagnosis.
From the QLQ-CIPN20, the mean score for sensory symptoms increased from 5 at baseline to 14 at the end of treatment, with higher scores representing increased symptomatology. At month 60 the mean score remained at 11, indicating a high level of persistent sensory neuropathy following R-CHOP chemotherapy.
Conclusion:
These results suggest that longer term QoL is not adversely affected by R-CHOP treatment in those who remain alive and disease-free after 5 years of follow-up. Symptoms from the disease itself appear to have a greater negative impact on QoL than R-CHOP treatment. These QoL results serve as a baseline for the assessment of novel interventions in patients receiving induction therapy for DLBCL.
Disclosures
Novak:Beigene: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees; Astra Zeneca: Membership on an entity's Board of Directors or advisory committees; Ideogen: Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pierre Fabre: Membership on an entity's Board of Directors or advisory committees; Celgene/BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees; Kyowa Kirin: Membership on an entity's Board of Directors or advisory committees; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees; H & O Communication: Honoraria. Burton:Roche: Consultancy. McKay:Abbvie, AstraZeneca, Beigene, Celgene/BMS, Epizyme, Gilead/Kite, Incyte, Janssen, Recordati Rare Diseases, Roche, Takeda: Consultancy; Gilead/Kite, Incyte, Janssen: Speakers Bureau. Campbell:Takeda: Honoraria; Abbvie: Honoraria; Novartis: Honoraria; Roche: Honoraria. Davies:Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Research support; Incyte: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees; GSK: Other: Research support; Ascerta Pharma/Astra Zeneca: Honoraria, Other: Research support; Kite, a Gilead company: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Research support; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Research support. Travel to scientific conferences, Research Funding; Celegne (a Bristol Myers Squibb company): Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel to scientific conferences, Research Funding. Johnson:Boehringer Ingelheim: Consultancy; BMS: Honoraria; Celgene: Honoraria; Genmab: Honoraria; Incyte: Honoraria; Kite Pharma: Honoraria; Kymera: Honoraria; MorphoSys: Honoraria; Novartis: Honoraria; Takeda: Honoraria; Oncimmune: Consultancy; Janssen: Consultancy; Epizyme: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.